Ambient Pressure Definition and Its Significance in Science
# Ambient Pressure Definition and Its Significance in Science
## What is Ambient Pressure?
Ambient pressure refers to the pressure of the surrounding environment at any given location. It is the force exerted by the weight of the atmosphere above a specific point on Earth’s surface or within any gaseous environment. Typically measured in units such as pascals (Pa), atmospheres (atm), or pounds per square inch (psi), ambient pressure varies depending on altitude, weather conditions, and other environmental factors.
At sea level, the standard ambient pressure is approximately 101.325 kilopascals (kPa), which is equivalent to 1 atmosphere (atm) or 14.696 psi. This value decreases as altitude increases because there is less atmospheric mass above the measurement point.
## How Ambient Pressure is Measured
Scientists and engineers use various instruments to measure ambient pressure:
– Barometers: Traditional mercury barometers measure atmospheric pressure by balancing it against a column of mercury
– Aneroid barometers: Use a flexible metal chamber that expands or contracts with pressure changes
– Digital pressure sensors: Modern electronic devices that convert pressure into electrical signals
## The Importance of Ambient Pressure in Scientific Applications
Ambient pressure plays a crucial role in numerous scientific fields:
### 1. Meteorology and Weather Forecasting
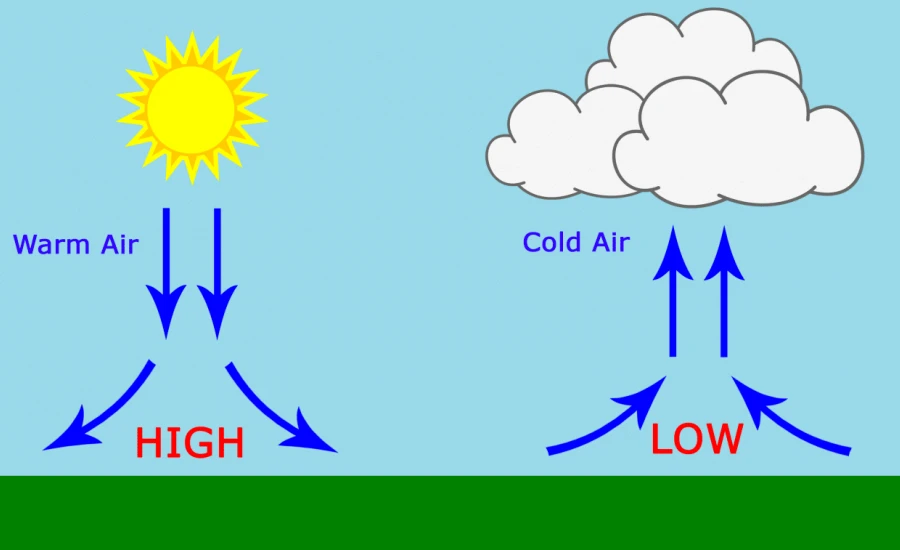
Atmospheric pressure variations are fundamental to weather patterns. Low-pressure systems typically bring stormy weather, while high-pressure systems are associated with clear skies. Meteorologists use pressure measurements to predict weather changes and track storm systems.
### 2. Aviation and Aerospace
Pilots and aerospace engineers must account for ambient pressure changes during flight. As aircraft ascend, the decreasing ambient pressure affects:
– Aircraft performance
– Cabin pressurization requirements
– Oxygen availability for passengers and crew
### 3. Chemistry and Physics
Many chemical reactions and physical processes are pressure-dependent. Scientists conducting experiments must consider ambient pressure or control it in laboratory settings. This is particularly important for:
– Gas law calculations
– Phase transitions
– Reaction kinetics
### 4. Medicine and Physiology
Human physiology is significantly affected by ambient pressure changes:
– Scuba divers must manage pressure changes to avoid decompression sickness
– High-altitude medicine studies the effects of low pressure on the human body
– Hyperbaric oxygen therapy uses increased pressure to enhance medical treatments
## Variations in Ambient Pressure
Ambient pressure isn’t constant and varies due to several factors:
– Altitude: Pressure decreases approximately 1 kPa for every 100 meters of elevation gain
– Weather systems: High and low pressure systems can cause variations of ±5% from standard pressure
– Temperature: Warm air is less dense than cold air, affecting local pressure
– Humidity: Water vapor content slightly influences atmospheric pressure
Understanding ambient pressure and its variations is essential for accurate scientific measurements, technological applications, and predicting natural phenomena. From weather forecasting to space exploration, this fundamental environmental parameter continues to play a vital role in advancing our understanding of the world around us.
Keyword: ambient pressure definition


